More than once you may have wondered why pool water turns greenish even when chlorine has been added. It’s simple: Pool water turns green because microscopic algae grow in it and give it that color. And they grow very easily and quickly. To avoid this, chlorine is added to the pools.
The incidence that I have encountered most frequently, especially in domestic pools and in areas with predominantly hard water (such as those in Mallorca) is that the pool, despite the addition of chlorine in some of its variants, does not lose its greenish color or does not acquire the desired transparency.
The explanation is as follows:
Chlorination is the most widely used water disinfection procedure because of its ease of use, broad boicidal spectrum and cost-effectiveness.
When chlorine is added to water, in any of its most common forms, whether as sodium hypochlorite (bleach), trichloroisocyanurates (chlorine powder or granules), chlorine gas or chlorine formation by salt hydrolysis, the chlorine will converge in the same chemical form: hypochlorous acid (HOCl).
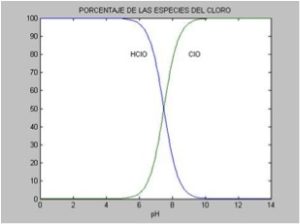
The key is that hypochlorous acid (HOCl) has a much higher biocidal power than the hypochlorite ion, which is formed as the pH rises, so that at pH 8.0, only 18% of the chlorine dissolved in the water is found in the most active form as a disinfectant. This percentage drops to 6% at pH 8.5 and is negligible at pH 9.0.
The conclusion is that, before adding chlorine, it is crucial to regulate the pH, so that it is ideally around 7.2-7-6 and always below 8.0.
Therefore, it is not only a matter of adding chlorine, but of making sure beforehand that the pH is at the ideal values. Otherwise we are wasting the disinfectant product.
J.M.Berrio

 English
English
 Português
Português  Português
Português 