If this holiday you find a salty taste in pool water, it is likely that the method of used use to generate free residual chlorine is salt electrolysis.
Why is it salty?
In the electrolysis there is no active chemical added to water, but is added common salt (sodium chloride) as a reagent to create free residual chlorine that has the biocide (disinfectant) effect.
Salt concentrations which are added to the pool water varies around 5 g / l, whereas seawater has a salt concentration around 35 g / l (that is 7 times more concentrated)
How does it work?
Water, once it has reached that salt concentration, and due the flow that occurs for the filtration process, passes through a device with electrically polarized electrodes so that electrolysis happen.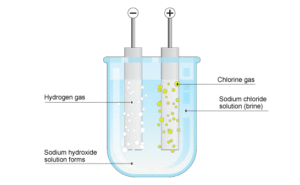
This process generates sodium (Na) and chlorine gas (Cl2). Chlorine gas reacts with water to form hypochlorous acid (HClO), resulting in residual free chlorine. This is the molecule that disinfects the pool because of his biocide power
Free residual chlorine is the same molecule you get when you add chlorine tablets or granular form (tricloroisocianurates) or in liquid (sodium hypochlorite).
Pros and cons.
This system has the advantage not to manipulate directly a hazardous biocide, but a much more innocuous substance as is common salt, but we must not neglect the pH control is even tighter (the recommended range is between 7.1 and 7.3).
Another advantage over the use of chlorine tablets, granules or powder (tricloroisocianurates) is avoiding letting cyanuric acid in the pool as a residue. Cyanuric acid is used as a stabilizer for chlorine and chlorine used in granular or powder. If cyanuric acid levels are high (greater than 100 ppm) can affect the biocidal capability of chlorine and also have an impacto over water clarity.
Since the use of this system is advantageous, it is not surprising that this system is increasingly widespread. Therefore, if the pool water is somewhat salty, do not be surprised …
HS consulting team

 English
English
 Português
Português  Português
Português 